LipidSol®
Lipid Nanoparticle Technology
LipidSol is a proprietary lipid nanoparticle technology developed by Amachems Pharmaceuticals®. Composed of lipid-based assemblies, LipidSol works to encapsulate small and large molecules as well as biologics in nanoparticle structures. This includes the encapsulation of:
Lipid nanoparticle technology is nanotechnology designed to optimize the use and/or performance of lipid nanoparticles (LNPs) in pharmaceutical drug products.
What Are Lipid Nanoparticles?
Lipid nanoparticles, quite simply, are nanoparticles composed of lipids. Essentially spherical vesicles made of ionizable lipids, lipid nanoparticles play a key role in effectively protecting and transporting mRNA to cells.
Advantages of Lipid Nanoparticles
Lipid nanoparticles present exciting opportunities to improve bioavailability, enhance solubility, and provide controlled, site-specific drug delivery, with applications for vaccines, cancer treatments, gene therapy products, and more.
LipidSol Lipid Nanoparticle Technology: How It Works
LipidSol enables the encapsulation of drugs in lipid nanoparticles (LNPs) by utilization of fatty acids and polar headgroup entities commercially available and listed in the FDA inactive ingredient database (FDA IID) or accessible to a novel lipid library.
Applications of LipidSol LNP Technology
Following are example assembly designs for Amachems’s proprietary LipidSol LNP technology, based on varied lipid structures and compositions:
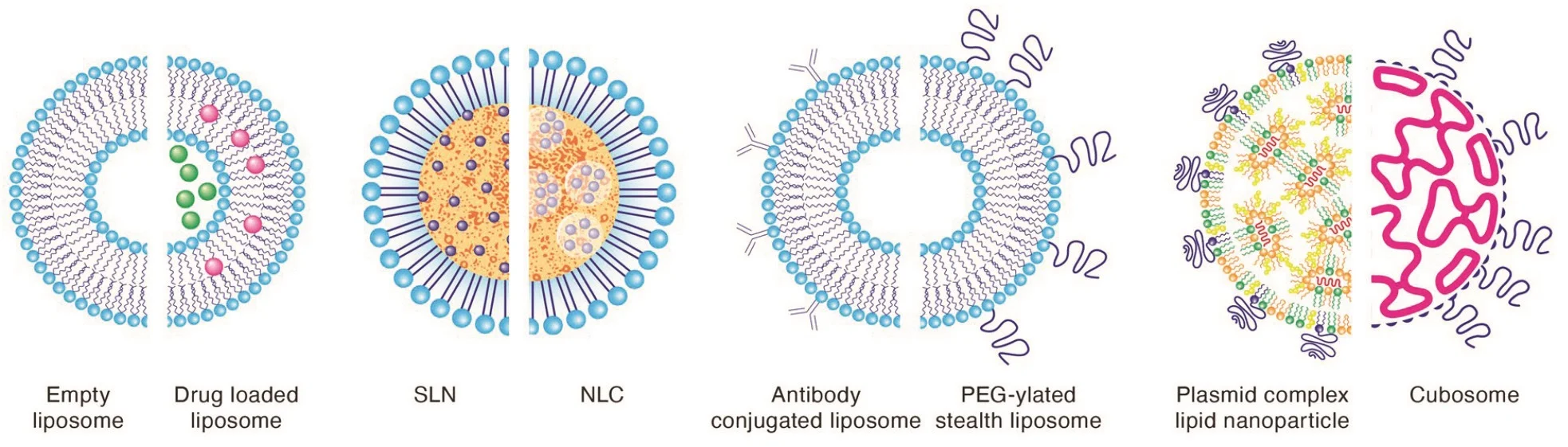
- Integrated with options to design with PEGylated phospholipids (stealth liposomes), LipidSol can trigger longer circulation times without being taken up by a reticuloendothelial system (RES). The result is the continued delivery of molecules for an extended period, especially those applicable to long-acting injectables in LNPs.
- LipidSol designed with antibodies (immunogenic liposomes) can target specific diseased tissues. Therefore, they reduce the eminent toxicity to healthier cells and can safely be administered to enhance drug efficacy.
- When LipidSol is designed with cationic phospholipids, it can effectively address complex synthetic lipid carriers of DNA, such as lipoplexes, to protect and transport DNA and mRNA to targeted cells.
- LipidSol can also be used as non-lamellar structured nanocarriers (cubosomes) and stabilized with a polymeric outer corona surface. It possesses significantly higher surface area for loading small molecules and proteins than typical liposomes.
Benefits of LipidSol LNP Technology
LipidSol lipid nanoparticle technology can enhance the loading and efficient drug delivery of small and large molecules by inhalation and injectable (intravenous, intramuscular, and subcutaneous) routes of administration. It is primarily aimed at addressing the delivery issues of biologics, plasmids DNA, RNAs, peptides, and small molecules by enhancing their efficacy and safe administration for the treatment of life-threatening ailments and life cycle management.
For the best design and encapsulation efficacy of modalities, the LNP lipid structures, size, charge, and morphology play key roles in protecting and carrying the payload to target sites via systemic circulation. Longer circulation means the LNPs are stable and prevented from being captured by Kupffer’s cells in blood streams, providing a better mechanism for the controlled delivery of modalities through an extended period.
Why Choose Amachems for Your LNP Needs
To better deliver the payload into the target cell, cationic lipids sourced from commercially available Generally Recognized As Safe (GRAS) material or from a proprietary lipid library are accessible to Amachems for use in LNP formulations. In addition, Amachems has state-of-art research, scale-up, and cGMP sterile manufacturing capabilities for LNPs that other pharmaceutical contract development and manufacturing organizations (CDMOs) do not.
In conjunction to lipid analytical method development, aseptic processing and fill & finish, and ISO 5/7 clean room facilities, our facilities offer cutting-edge equipment ready to use for different LNP processes, including:
- Solvent injection
- Film rehydration
- Nanoassembly
- Microfluidization
- Tangential flow filtration (TFF)
- Lyophilization
Our main equipment for LNPs includes a microfluidic chip mixer, NanoAssemblr, high shear microfluidizer, high pressure homogenizer, extruder, and rotavapor, among others, ensuring Amachems’s drug development partners benefit from a top-down and bottom-up outsourcing approach to formulation development and pharmaceutical contract manufacturing services.
Want to learn more about
Amachems's expertise?
Get up-to-date information
.png)
.png)
.png)
.png)
