AmorSol®
Amorphous Solid Dispersion Technology
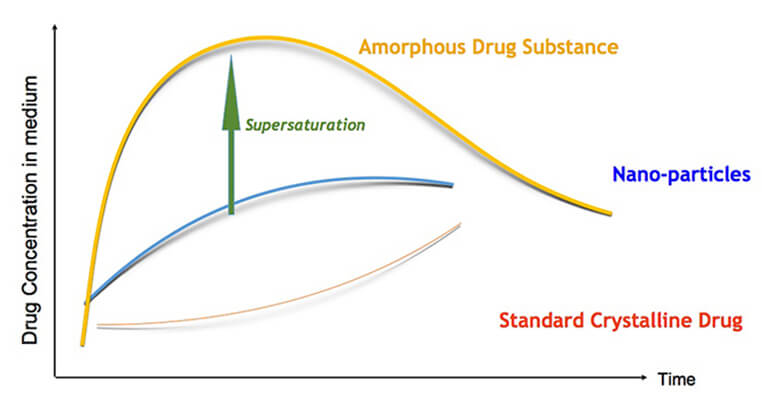
AmorSol is a technology for the production of amorphous solid dispersions. Formulation of a drug substance in amorphous form greatly improves its solubility and dissolution kinetics, and has become a standard formulation technique in the pharmaceutical industry for reaching bioavailability goals for poorly water soluble drugs. Amorphous drug substance forms are meta-stable, and can be stabilized by using various polymers to restrict or delay recrystallization of the drug.
Solid dispersions are used to increase the dissolution rate, a limiting factor for the absorption of many compounds in development today. A solid dispersion is a dispersion of the compound in a solid, inert matrix carrier, generally a poorly water soluble drug in a hydrophilic carrier (e.g., a polymer).
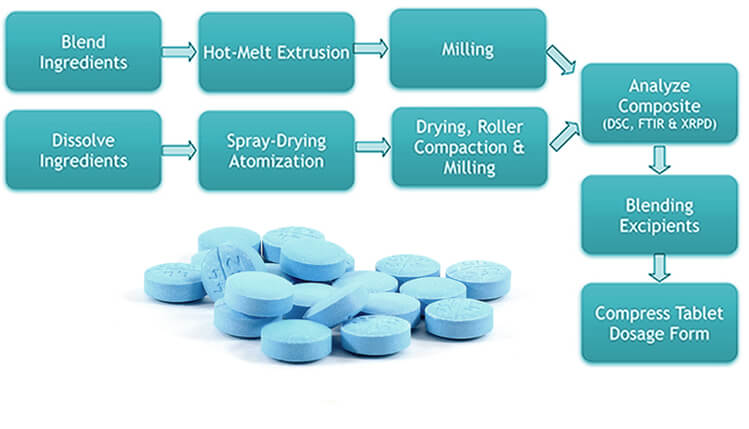
Amorphous solid dispersions can be prepared by either a spray-drying (i.e., evaporation of a solvent solution of the drug and polymer), or by hot melt extrusion (i.e., melting of the drug substance with a polymer followed by co-extrusion). For characterization of solid dispersions, Amachems® uses state-of-the-art analytical sciences such as differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), FTIR, and other solid state characterization techniques.
Amachems’s amorphous solid dispersion technology can enable pharmaceutical products with enhanced bioavailability, reduced food-effect and more rapid onset-of-action.
PH INDEPENDENT DISSOLUTION CASE STUDY
Often oral dosage forms of poorly soluble drugs exhibit a pH-sensitive dissolution. In addition to the variation of stomach pH with vs. without food, a high-fat meal can exacerbate the difference in bioavailability when a medication is taken with food vs. without. Amachems has developed a formulation of prasugrel, a platelet aggregation inhibitor, that overcomes the food effect associated with the branded product Effient®. Prasugrel has a water solubility of only 2µg/ml, and as a weakly basic compound (pKa=5.48) has a highly pH dependent solubility profile.
As shown in Figure 1, the dissolution of prasugrel from a conventional solid formulation of prasugrel at pH 1.2 (fasted) and pH 6.8 (fed) are widely different. The difference in prasugrel dissolution can be as much as 400 fold between pH 1.2 and 6.8.
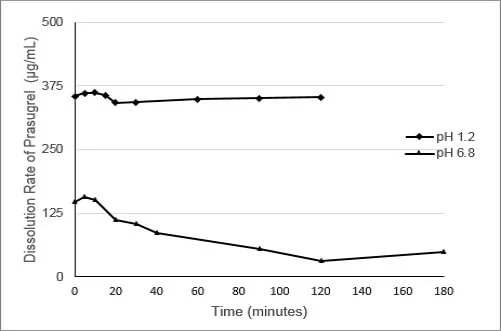
Figure 1: Dissolution of Prasugrel from a Conventional Solid Dosage Form
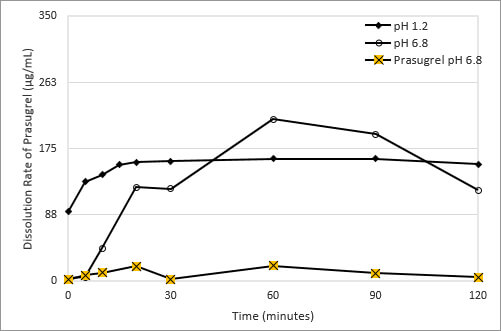
Figure 2: Dissolution of Prasugrel from Amachems's Amorphous Solid Dispersion Dosage Form
Using a blend of polymeric excipients having different water and pH solubility properties, Amachems formulated an amorphous solid dispersion composition of prasugrel that overcomes this pH-based solubility dependence. The drug substance is dissolved in a solvent, combined with the polymers, the solvent is evaporated, and the homogenous mixture of drug and polymer is processed into a powder suitable for tabletting. This formulation significantly reduces the pH-based solubility of prasugrel at pH 1.2 and 6.8 as shown in Figure 2.
Amachems specializes in the application of nano-technology approaches to develop novel pharmaceutical products. Our suite of state-of-the-art technologies for formulating poorly water-soluble compounds provides sophisticated formulation options for our clients. Contact us today for more information on how our formulations can enable your pharmaceutical product.
Want to learn more about
Amachems's expertise?
Get up-to-date information
.png)
.png)
.png)
.png)
